Deciphering molecular chirality with a new ultrafast imaging technique
An international team of researchers has identified a phenomenon that could allow them to image molecular chirality with extremely high temporal resolution.
Chirality and Its Challenges in Ultrafast Imaging
Molecular dynamics unfold on incredibly short time scales. To witness and comprehend how electrons and nuclei rearrange during a chemical reaction, researchers must capture ultrafast snapshots. Short laser pulses provide the necessary time resolution, but interpreting and reconstructing the molecular dynamics from the resulting images and measurements poses a significant challenge, especially for complex and non-symmetric molecules with multiple nuclei.
Now, a particularly interesting and important property that emerges with molecules holding more than three nuclei is the known as chirality. Chirality (or handedness) is fundamental in chemistry because it plays an important role in the recognition phenomenon between biologically active molecules, and it can modify the outcome of a chemical reaction depending on the handedness of molecular structure.
The phenomenon of chirality relates to the three-dimensional structure of molecules. You cannot superpose a chiral molecule and its mirror image through any combination of rotations and translations (similar to the right and left hand). Furthermore, chiral molecules rotate the plane of polarized light when light shines on them. Scientists call this degree of rotation specific rotation or optical rotation. However, the inherent weakness of this well-known optical rotation effect makes it difficult to extend to the ultrafast regime. These challenges hinder scientists from imaging molecular chirality in the ultrafast regime, limiting our ability to image and interpret electron dynamics in complex and relevant biological molecules.
Pioneering Research: Imaging Molecular Chirality with Ultrafast Resolution
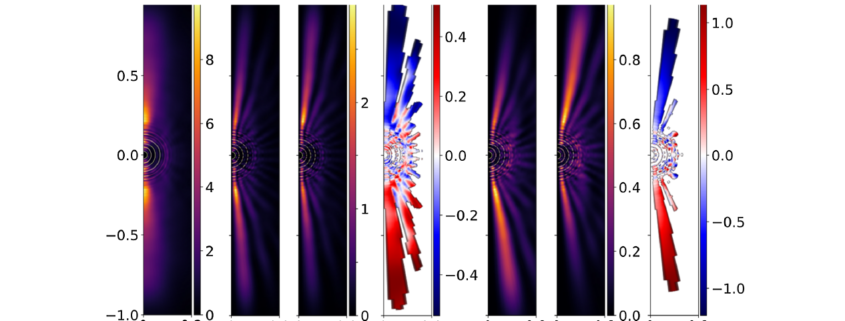
Developing methods to image molecular chirality with high temporal resolution is a key step towards understanding the molecular dynamics of the not-so-simple systems. This is what was achieved in a recent study published in Physical Review Letters, where ICFO researchers Xavier Barcons (now at the German Aerospace Center), Andres Ordoñez, and Andrew Maxwell (now at Aarhus University), led by ICREA Prof at ICFO and Dynamite project coordinator Maciej Lewenstein have identified a phenomenon that could allow them to image molecular chirality with extremely high temporal resolution (on the order of 10-16 s), which depends on a fundamental property of electrons, unexploited in this way until now.
In their theoretical approach, they considered the photoionization of a chiral molecule using a short, intense infrared pulse. As Andres Ordoñez explains, “The individual photons in this pulse lack sufficient energy to ionize the molecule, but the pulse’s intensity allows the molecule to absorb multiple photons simultaneously. The ionization is better understood as the pulse’s high electric field ripping the electron away from the molecule. Prior to this, the chiral nuclear arrangement determines the electron cloud’s shape.”
Upon separation from the molecule, the electron cloud retains an imprint of its initial state and the chirality of the molecule. This structure can be quantified in terms of the orbital angular momentum (OAM) of the electron cloud and the direction in which it moves away from the molecule. The team discovered that upon ionizing the molecule with linearly polarized light, the molecular chirality is imprinted in the OAM-helicity of the photoelectron. They observed that emitted electrons carry information about the chirality of their originating molecule through the retrieved OAM information, a fundamental phenomenon previously unknown to them.
Implications and Future Directions
The results of this work prove it to be the first study that explores the role that the orbital angular momentum (OAM) of free electrons play in the context of photoionization of chiral molecules. This will not only mark the commencement of collaborations aimed to design an attosecond experiment, where the scientists will seek to measure the electron’s OAM and demonstrate their key theoretical results. It will also provide insights on an improved description of the chiral molecule, which at the moment relies on a toy model that captures the essence of the phenomenon, but which does not include important aspects such as the anisotropy of the molecular potential, or recollision scenarios that lead to interesting strong-field phenomena such as high-harmonic generation and light-induced electron diffraction, among others.
As Maciej Lewenstein concludes, “While it is way too early to claim that the particular effect that we found will revolutionize sectors, such as the pharmaceutical sector, we are certain that, at the very least, it will inspire further research into molecular chirality.
Why Chirality is so important
Chiral molecules are ubiquitous in nature, in particular in biological systems, where, for example, most aminoacids and sugars are chiral. Molecular handedness is important because it determines how a chiral molecule reacts with another chiral molecule. Thus, although the left and right handed versions of a chiral molecule look very similar to each other (they are just mirror images of each other, like hands), they can behave rather differently in certain situations. The most striking examples of this occur in the pharmaceutical sector, where chirality has played a fundamental role in the development of drugs, as one handedness may have a positive effect while the opposite handedness may be extremely toxic. Therefore, developing new methods that extend our ability to measure molecular chirality undoubtedly has an impact on society.
Original article
Planas, X. B., Ordóñez, A., Lewenstein, M., & Maxwell, A. S. (2022). Ultrafast Imaging of Molecular Chirality with Photoelectron Vortices. Physical Review Letters, 129(23), 233201. https://doi.org/10.1103/PhysRevLett.129.233201

 This project was funded within the QuantERA II Programme that has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 101017733
This project was funded within the QuantERA II Programme that has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 101017733
